Designing hydrogels for controlled drug delivery
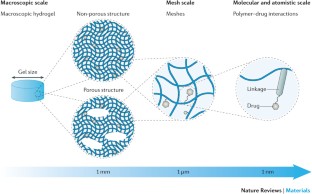
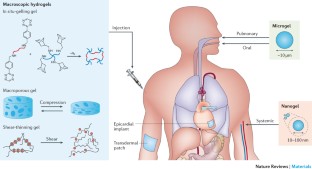
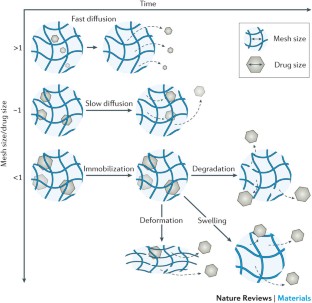
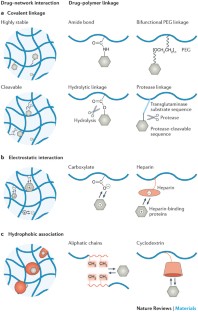
Hydrogel delivery systems can leverage therapeutically beneficial outcomes of drug delivery and have found clinical use. Hydrogels can provide spatial and temporal control over the release of various therapeutic agents, including small-molecule drugs, macromolecular drugs and cells. Owing to their tunable physical properties, controllable degradability and capability to protect labile drugs from degradation, hydrogels serve as a platform on which various physiochemical interactions with the encapsulated drugs occur to control drug release. In this Review, we cover multiscale mechanisms underlying the design of hydrogel drug delivery systems, focusing on physical and chemical properties of the hydrogel network and the hydrogel–drug interactions across the network, mesh and molecular (or atomistic) scales. We discuss how different mechanisms interact and can be integrated to exert fine control in time and space over drug presentation. We also collect experimental release data from the literature, review clinical translation to date of these systems and present quantitative comparisons between different systems to provide guidelines for the rational design of hydrogel delivery systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
133,45 € per year
only 11,12 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Model-based modular hydrogel design
Article 26 March 2024
Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions
Article Open access 01 July 2024
Influence of drug and polymer molecular weight on release kinetics from HEMA and HPMA hydrogels
Article Open access 04 October 2023
References
- Langer, R. Drug delivery and targeting. Nature392, 5–10 (1998). CASGoogle Scholar
- Hoare, T. R. & Kohane, D. S. Hydrogels in drug delivery: progress and challenges. Polymer49, 1993–2007 (2008). CASGoogle Scholar
- Liechty, W. B., Kryscio, D. R., Slaughter, B. V. & Peppas, N. A. Polymers for drug delivery systems. Ann. Rev. Chem. Biomol. Eng.1, 149–173 (2010). CASGoogle Scholar
- Cohen, J. IL-12 deaths: explanation and a puzzle. Science270, 908 (1995). CASGoogle Scholar
- Florence, A. T. & Jani, P. U. Novel oral drug formulations. Drug Safety10, 233–266 (1994). CASGoogle Scholar
- Ashley, G. W., Henise, J., Reid, R. & Santi, D. V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl Acad. Sci. USA110, 2318–2323 (2013). This study features cleavable covalent linkages with tunable half-lives over a wide range and demonstrates different drug release kinetics by orchestrating the rates of bulk erosion and linkage cleavage independently.CASGoogle Scholar
- Tiwari, G. et al. Drug delivery systems: an updated review. Int. J. Pharm. Investig.2, 2–11 (2012). Google Scholar
- Tibbitt, M. W., Dahlman, J. E. & Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc.138, 704–717 (2016). CASGoogle Scholar
- Calvert, P. Hydrogels for soft machines. Adv. Mater.21, 743–756 (2009). CASGoogle Scholar
- Arakaki, K. et al. Artificial cartilage made from a novel double-network hydrogel: in vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J. Biomed. Mater. Res. Part A93A, 1160–1168 (2010). CASGoogle Scholar
- Li, J., Illeperuma, W. R., Suo, Z. & Vlassak, J. J. Hybrid hydrogels with extremely high stiffness and toughness. ACS Macro Lett.3, 520–523 (2014). CASGoogle Scholar
- Bodugoz-Senturk, H., Macias, C. E., Kung, J. H. & Muratoglu, O. K. Poly(vinyl alcohol)–acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials30, 589–596 (2009). CASGoogle Scholar
- Su, J., Hu, B.-H., Lowe, W. L., Kaufman, D. B. & Messersmith, P. B. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials31, 308–314 (2010). This study demonstrates a synergy between adhesion ligands and cytokine-suppressive peptides, which improves viability of insulin-secreting cells in the presence of pro-inflammatory cytokines.CASGoogle Scholar
- Reichert, J. M. Trends in development and approval times for new therapeutics in the United States. Nat. Rev. Drug Discov.2, 695–702 (2003). CASGoogle Scholar
- Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov.7, 21–39 (2008). CASGoogle Scholar
- Khan, T. A. & Peh, K. K. & Ch'ng, H. S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci.3, 303–311 (2000). CASGoogle Scholar
- Mahdavi, A. et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl Acad. Sci. USA105, 2307–2312 (2008). CASGoogle Scholar
- Di, J. et al. Stretch-triggered drug delivery from wearable elastomer films containing therapeutic depots. ACS Nano9, 9407–9415 (2015). CASGoogle Scholar
- Bessa, P. C., Casal, M. & Reis, R. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med.2, 81–96 (2008). CASGoogle Scholar
- Thorn, R., Greeman, J. & Austin, A. An in vitro study of antimicrobial activity and efficacy of iodine-generating hydrogel dressings. J. Wound Care15, 305 (2006). CASGoogle Scholar
- Momoh, F. U., Boateng, J. S., Richardson, S. C., Chowdhry, B. Z. & Mitchell, J. C. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int. J. Biol. Macromol.81, 137–150 (2015). CASGoogle Scholar
- Pandit, A., Ashar, R. & Feldman, D. The effect of TGF-β delivered through a collagen scaffold on wound healing. J. Invest. Surg.12, 89–100 (1999). CASGoogle Scholar
- Jayakumar, R., Prabaharan, M., Kumar, P. S., Nair, S. & Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv.29, 322–337 (2011). CASGoogle Scholar
- Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci.37, 106–126 (2012). CASGoogle Scholar
- Tellechea, A. et al. Alginate and DNA gels are suitable delivery systems for diabetic wound healing. Int. J. Low. Extrem. Wounds14, 146–153 (2015). CASGoogle Scholar
- Zhang, L., Chen, J. & Han, C. A multicenter clinical trial of recombinant human GM-CSF hydrogel for the treatment of deep second-degree burns. Wound Repair Regen.17, 685–689 (2009). Google Scholar
- Liu, W., Griffith, M. & Li, F. Alginate microsphere-collagen composite hydrogel for ocular drug delivery and implantation. J. Mater. Sci. Mater. Med.19, 3365–3371 (2008). CASGoogle Scholar
- Dash, A. & Cudworth, G. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods40, 1–12 (1998). CASGoogle Scholar
- Yu, L. & Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev.37, 1473–1481 (2008). CASGoogle Scholar
- Silva, E. A. & Mooney, D. J. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemost.5, 590–598 (2007). This study demonstrates the ability of needle-injectable alginate hydrogels to regulate the temporal and spatial presentation of VEGF for the treatment of ischaemic diseases in a rodent model.CASGoogle Scholar
- Silva, E. A., Kim, E.-S., Kong, H. J. & Mooney, D. J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc. Natl Acad. Sci. USA105, 14347–14352 (2008). CASGoogle Scholar
- Hiemstra, C. et al. In vitro and in vivo protein delivery from in situ forming poly(ethylene glycol)–poly(lactide) hydrogels. J. Control. Release119, 320–327 (2007). CASGoogle Scholar
- Jin, R. et al. Synthesis and characterization of hyaluronic acid–poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater.6, 1968–1977 (2010). CASGoogle Scholar
- Lim, D. W., Nettles, D. L., Setton, L. A. & Chilkoti, A. Rapid cross-linking of elastin-like polypeptides with (hydroxymethyl) phosphines in aqueous solution. Biomacromolecules8, 1463–1470 (2007). CASGoogle Scholar
- Wieduwild, R. et al. Minimal peptide motif for non-covalent peptide–heparin hydrogels. J. Am. Chem. Soc.135, 2919–2922 (2013). CASGoogle Scholar
- Kiick, K. L. Peptide-and protein-mediated assembly of heparinized hydrogels. Soft Matter4, 29–37 (2008). CASGoogle Scholar
- Ishii, S., Kaneko, J. & Nagasaki, Y. Development of a long-acting, protein-loaded, redox-active, injectable gel formed by a polyion complex for local protein therapeutics. Biomaterials84, 210–218 (2016). CASGoogle Scholar
- Desai, R. M., Koshy, S. T., Hilderbrand, S. A., Mooney, D. J. & Joshi, N. S. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials50, 30–37 (2015). CASGoogle Scholar
- Jewett, J. C. & Bertozzi, C. R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev.39, 1272–1279 (2010). CASGoogle Scholar
- DeForest, C. A. & Anseth, K. S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem.3, 925–931 (2011). This study demonstrates the synthesis of light-responsive hydrogels, which enable photoconjugation of peptides and cell encapsulation, using a combination of bio-orthogonal click chemistries and photoreactions.CASGoogle Scholar
- Cao, Y. et al. Poly(N-isopropylacrylamide)–chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release120, 186–194 (2007). CASGoogle Scholar
- Mortensen, K. & Pedersen, J. S. Structural study on the micelle formation of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer in aqueous solution. Macromolecules26, 805–812 (1993). CASGoogle Scholar
- Kwon, D. Y. et al. Synergistic anti-tumor activity through combinational intratumoral injection of an in-situ injectable drug depot. Biomaterials85, 232–245 (2016). Google Scholar
- Davidorf, F. H. et al. Ocular toxicity of vitreal pluronic polyol F-127. Retina10, 297–300 (1990). CASGoogle Scholar
- Censi, R. et al. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release140, 230–236 (2009). CASGoogle Scholar
- van de Wetering, P., Metters, A. T., Schoenmakers, R. G. & Hubbell, J. A. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J. Control. Release102, 619–627 (2005). CASGoogle Scholar
- Guvendiren, M., Lu, H. D. & Burdick, J. A. Shear-thinning hydrogels for biomedical applications. Soft Matter8, 260–272 (2012). CASGoogle Scholar
- Altunbas, A., Lee, S. J., Rajasekaran, S. A., Schneider, J. P. & Pochan, D. J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials32, 5906–5914 (2011). CASGoogle Scholar
- Rajagopal, K. & Schneider, J. P. Self-assembling peptides and proteins for nanotechnological applications. Curr. Opin. Struct. Biol.14, 480–486 (2004). CASGoogle Scholar
- Haines-Butterick, L. et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl Acad. Sci. USA104, 7791–7796 (2007). CASGoogle Scholar
- Yan, C. et al. Injectable solid hydrogel: mechanism of shear-thinning and immediate recovery of injectable β-hairpin peptide hydrogels. Soft Matter6, 5143–5156 (2010). CASGoogle Scholar
- Haines-Butterick, L. A., Salick, D. A., Pochan, D. J. & Schneider, J. P. In vitro assessment of the pro-inflammatory potential of β-hairpin peptide hydrogels. Biomaterials29, 4164–4169 (2008). CASGoogle Scholar
- Micklitsch, C. M. et al. Zinc-triggered hydrogelation of a self-assembling β-hairpin peptide. Angew. Chem. Int. Ed.123, 1615–1617 (2011). Google Scholar
- Rowan, S. J., Cantrill, S. J., Cousins, G. R., Sanders, J. K. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed.41, 898–952 (2002). Google Scholar
- McKinnon, D. D., Domaille, D. W., Cha, J. N. & Anseth, K. S. Bis-aliphatic hydrazone-linked hydrogels form most rapidly at physiological pH: identifying the origin of hydrogel properties with small molecule kinetic studies. Chem. Mater.26, 2382–2387 (2014). CASGoogle Scholar
- Jin, Y., Yu, C., Denman, R. J. & Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev.42, 6634–6654 (2013). CASGoogle Scholar
- Yesilyurt, V. et al. Injectable self-healing glucose-responsive hydrogels with pH-regulated mechanical properties. Adv. Mater.28, 86–91 (2016). CASGoogle Scholar
- Plieva, F. M., Galaev, I. Y., Noppe, W. & Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol.16, 543–551 (2008). CASGoogle Scholar
- Sheridan, M., Shea, L., Peters, M. & Mooney, D. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control. Release64, 91–102 (2000). CASGoogle Scholar
- Zhou, S., Bismarck, A. & Steinke, J. H. Ion-responsive alginate based macroporous injectable hydrogel scaffolds prepared by emulsion templating. J. Mater. Chem. B1, 4736–4745 (2013). CASGoogle Scholar
- Hassan, C. M. & Peppas, N. A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules33, 2472–2479 (2000). CASGoogle Scholar
- Huebsch, N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater.14, 1269–1277 (2015). CASGoogle Scholar
- Bencherif, S. A. et al. Injectable preformed scaffolds with shape-memory properties. Proc. Natl Acad. Sci. USA109, 19590–19595 (2012). CASGoogle Scholar
- Bencherif, S. A. et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun.6, 7556 (2015). CASGoogle Scholar
- Alexis, F., Pridgen, E., Molnar, L. K. & Farokhzad, O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm.5, 505–515 (2008). CASGoogle Scholar
- Mitragotri, S. & Lahann, J. Physical approaches to biomaterial design. Nat. Mater.8, 15–23 (2009). CASGoogle Scholar
- Euliss, L. E., DuPont, J. A., Gratton, S. & DeSimone, J. Imparting size, shape, and composition control of materials for nanomedicine. Chem. Soc. Rev.35, 1095–1104 (2006). CASGoogle Scholar
- Gratton, S. E. et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA105, 11613–11618 (2008). CASGoogle Scholar
- Merkel, T. J. et al. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J. Control. Release162, 37–44 (2012). CASGoogle Scholar
- Ginn, S. L., Alexander, I. E., Edelstein, M. L., Abedi, M. R. & Wixon, J. Gene therapy clinical trials worldwide to 2012 – an update. J. Gene Med.15, 65–77 (2013). CASGoogle Scholar
- Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol.2, 751–760 (2007). CASGoogle Scholar
- Vinogradov, S. V., Bronich, T. K. & Kabanov, A. V. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev.54, 135–147 (2002). CASGoogle Scholar
- Vicent, M. J. & Duncan, R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol.24, 39–47 (2006). CASGoogle Scholar
- Li, J. et al. Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J. Am. Chem. Soc.137, 1412–1415 (2015). A modular design of DNA nanogels for gene therapy was presented that can incorporate different functional elements to target specific cells and release therapeutic genes inside cells.CASGoogle Scholar
- Oh, J. K., Drumright, R., Siegwart, D. J. & Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci.33, 448–477 (2008). CASGoogle Scholar
- Rolland, J. P. et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc.127, 10096–10100 (2005). This study presents a versatile top-down technique for the fabrication of nanogels and microgels, which provides fine control over particle size and shape, and is compatible with various therapeutic agents.CASGoogle Scholar
- Perry, J. L. et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett.12, 5304–5310 (2012). CASGoogle Scholar
- Dunn, S. S. et al. Reductively responsive siRNA-conjugated hydrogel nanoparticles for gene silencing. J. Am. Chem. Soc.134, 7423–7430 (2012). CASGoogle Scholar
- Peppas, N. A. & Sahlin, J. J. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials17, 1553–1561 (1996). CASGoogle Scholar
- Chaturvedi, M., Kumar, M. & Pathak, K. A review on mucoadhesive polymer used in nasal drug delivery system. J. Adv. Pharm. Technol. Res.2, 215 (2011). CASGoogle Scholar
- Reece, T. B., Maxey, T. S. & Kron, I. L. A prospectus on tissue adhesives. Am. J. Surg.182, S40–S44 (2001). Google Scholar
- Xu, J., Strandman, S., Zhu, J. X., Barralet, J. & Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials37, 395–404 (2015). CASGoogle Scholar
- Nho, Y.-C., Park, J.-S. & Lim, Y.-M. Preparation of poly(acrylic acid) hydrogel by radiation crosslinking and its application for mucoadhesives. Polymers6, 890–898 (2014). Google Scholar
- Bhattarai, N., Gunn, J. & Zhang, M. Chitosan-based hydrogels for controlled, localizeddrug delivery. Adv. Drug Deliv. Rev.62, 83–99 (2010). CASGoogle Scholar
- Ponchel, G. & Irache, J.-M. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev.34, 191–219 (1998). CASGoogle Scholar
- Shojaei, A. H., Paulson, J. & Honary, S. Evaluation of poly(acrylic acid-co-ethylhexyl acrylate) films for mucoadhesive transbuccal drug delivery: factors affecting the force of mucoadhesion. J. Control. Release67, 223–232 (2000). CASGoogle Scholar
- Das Neves, J. & Bahia, M. Gels as vaginal drug delivery systems. Int. J. Pharm.318, 1–14 (2006). CASGoogle Scholar
- Luppi, B. et al. Novel mucoadhesive nasal inserts based on chitosan/hyaluronate polyelectrolyte complexes for peptide and protein delivery. J. Pharm. Pharmacol.61, 151–157 (2009). CASGoogle Scholar
- Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science318, 426–430 (2007). CASGoogle Scholar
- Lee, B. P., Messersmith, P. B., Israelachvili, J. N. & Waite, J. H. Mussel-inspired adhesives and coatings. Ann. Rev. Mater. Res.41, 99 (2011). CASGoogle Scholar
- Brubaker, C. E., Kissler, H., Wang, L.-J., Kaufman, D. B. & Messersmith, P. B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials31, 420–427 (2010). CASGoogle Scholar
- Nafea, E., Marson, A., Poole-Warren, L. & Martens, P. Immunoisolating semi-permeable membranes for cell encapsulation: focus on hydrogels. J. Control. Release154, 110–122 (2011). CASGoogle Scholar
- Lake, G. J. & Thomas, A. G. Strength of highly elastic materials. Proc. R. Soc. A300, 108–119 (1967). CASGoogle Scholar
- Kong, H. J., Wong, E. & Mooney, D. J. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules36, 4582–4588 (2003). CASGoogle Scholar
- Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater.15, 1155–1158 (2003). CASGoogle Scholar
- Sun, J. Y. et al. Highly stretchable and tough hydrogels. Nature489, 133–136 (2012). CASGoogle Scholar
- Lin, C.-C. & Metters, A. T. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv. Drug Deliv. Rev.58, 1379–1408 (2006). CASGoogle Scholar
- Burczak, K., Fujisato, T., Hatada, M. & Ikada, Y. Protein permeation through poly(vinyl alcohol) hydrogel membranes. Biomaterials15, 231–238 (1994). CASGoogle Scholar
- Dubrovskii, S. A. & Rakova, G. V. Elastic and osmotic behavior and network imperfections of nonionic and weakly ionized acrylamide-based hydrogels. Macromolecules30, 7478–7486 (1997). CASGoogle Scholar
- Sakai, T. et al. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules41, 5379–5384 (2008). CASGoogle Scholar
- Lee, K. Y. & Mooney, D. J. Hydrogels for tissue engineering. Chem. Rev.101, 1869–1880 (2001). CASGoogle Scholar
- Vermonden, T., Censi, R. & Hennink, W. E. Hydrogels for protein delivery. Chem. Rev.112, 2853–2888 (2012). CASGoogle Scholar
- Young, M., Carroad, P. & Bell, R. Estimation of diffusion coefficients of proteins. Biotechnol. Bioeng.22, 947–955 (1980). CASGoogle Scholar
- Brazel, C. S. & Peppas, N. A. Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm.49, 47–58 (2000). CASGoogle Scholar
- Lin, Y.-H., Liang, H.-F., Chung, C.-K., Chen, M.-C. & Sung, H.-W. Physically crosslinked alginate/N,O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials26, 2105–2113 (2005). CASGoogle Scholar
- Amsden, B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules31, 8382–8395 (1998). CASGoogle Scholar
- MacArthur, J. W. Jr. et al. Sustained release of engineered stromal cell-derived factor 1-α from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation128, S79–S86 (2013). CASGoogle Scholar
- Boontheekul, T., Kong, H. J. & Mooney, D. J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials26, 2455–2465 (2005). CASGoogle Scholar
- O'shea, T. M., Aimetti, A. A., Kim, E., Yesilyurt, V. & Langer, R. Synthesis and characterization of a library of in-situ curing, nonswelling ethoxylated polyol thiol-ene hydrogels for tailorable macromolecule delivery. Adv. Mater.27, 65–72 (2015). CASGoogle Scholar
- Ishihara, M. et al. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. A64, 551–559 (2003). Google Scholar
- Lutolf, M. et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA100, 5413–5418 (2003). CASGoogle Scholar
- Um, S. H. et al. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater.5, 797–801 (2006). CASGoogle Scholar
- Purcell, B. P. et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater.13, 653–661 (2014). This study features a biomolecule-responsive hydrogel that can degrade in response to MMPs and release drugs for the treatment of myocardial infarction.CASGoogle Scholar
- Fischel-Ghodsian, F., Brown, L., Mathiowitz, E., Brandenburg, D. & Langer, R. Enzymatically controlled drug delivery. Proc. Natl Acad. Sci. USA85, 2403–2406 (1988). CASGoogle Scholar
- Podual, K., Doyle, F. J. & Peppas, N. A. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J. Control. Release67, 9–17 (2000). CASGoogle Scholar
- Maitz, M. F. et al. Bio-responsive polymer hydrogels homeostatically regulate blood coagulation. Nat. Commun.4, 2168 (2013). Google Scholar
- Lin, K. Y., Lo, J. H., Consul, N., Kwong, G. A. & Bhatia, S. N. Self-titrating anticoagulant nanocomplexes that restore homeostatic regulation of the coagulation cascade. ACS Nano8, 8776–8785 (2014). CASGoogle Scholar
- Zhang, Y., Wang, R., Hua, Y., Baumgartner, R. & Cheng, J. Trigger-responsive poly(β-amino ester) hydrogels. ACS Macro Lett.3, 693–697 (2014). CASGoogle Scholar
- Tibbitt, M. W., Han, B. W., Kloxin, A. M. & Anseth, K. S. Synthesis and application of photodegradable microspheres for spatiotemporal control of protein delivery. J. Biomed. Mater. Res. A100, 1647–1654 (2012). Google Scholar
- Yan, B., Boyer, J.-C., Habault, D., Branda, N. R. & Zhao, Y. Near infrared light triggered release of biomacromolecules from hydrogels loaded with upconversion nanoparticles. J. Am. Chem. Soc.134, 16558–16561 (2012). CASGoogle Scholar
- Siepmann, J. & Göpferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev.48, 229–247 (2001). CASGoogle Scholar
- Yu, H., Lu, J. & Xiao, C. Preparation and properties of novel hydrogels from oxidized konjac glucomannan cross-linked chitosan for in vitro drug delivery. Macromol. Biosci.7, 1100–1111 (2007). CASGoogle Scholar
- Sawhney, A. S., Pathak, C. P. & Hubbell, J. A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(α-hydroxy acid) diacrylate macromers. Macromolecules26, 581–587 (1993). CASGoogle Scholar
- Ma, G., Miao, B. & Song, C. Thermosensitive PCL-PEG-PCL hydrogels: synthesis, characterization, and delivery of proteins. J. Appl. Polym. Sci.116, 1985–1993 (2010). CASGoogle Scholar
- van de Manakker, F. et al. Protein-release behavior of self-assembled PEG–β-cyclodextrin/PEG–cholesterol hydrogels. Adv. Func. Mater.19, 2992–3001 (2009). CASGoogle Scholar
- Brannonpeppas, L. & Peppas, N. A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci.46, 715–722 (1991). CASGoogle Scholar
- Hong, W., Zhao, X., Zhou, J. & Suo, Z. A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. Phys. Solids56, 1779–1793 (2008). CASGoogle Scholar
- Hirokawa, Y. & Tanaka, T. Volume phase-transition in a nonionic gel. J. Chem. Phys.81, 6379–6380 (1984). Google Scholar
- Obaidat, A. A. & Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials18, 801–806 (1997). CASGoogle Scholar
- Kokufata, E., Zhang, Y.-Q. & Tanaka, T. Saccharide-sensitive phase transition of a lectin-loaded gel. Nature351, 302–304 (1991). CASGoogle Scholar
- Zhang, S. et al. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater.14, 1065–1071 (2015). CASGoogle Scholar
- Ohmine, I. & Tanaka, T. Salt effects on the phase-transition of ionic gels. J. Chem. Phys.77, 5725–5729 (1982). CASGoogle Scholar
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release92, 1–17 (2003). CASGoogle Scholar
- Mumper, R. J., Huffman, A. S., Puolakkainen, P. A., Bouchard, L. S. & Gombotz, W. R. Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): stabilization of TGF-β1 by the addition of polyacrylic acid within acid-treated beads. J. Control. Release30, 241–251 (1994). CASGoogle Scholar
- Kanamala, M., Wilson, W. R., Yang, M., Palmer, B. D. & Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials85, 152–167 (2016). CASGoogle Scholar
- Shirakura, T., Kelson, T. J., Ray, A., Malyarenko, A. E. & Kopelman, R. Hydrogel nanoparticles with thermally controlled drug release. ACS Macro Lett.3, 602–606 (2014). CASGoogle Scholar
- Ankareddi, I. & Brazel, C. S. Synthesis and characterization of grafted thermosensitive hydrogels for heating activated controlled release. Int. J. Pharm.336, 241–247 (2007). CASGoogle Scholar
- Huebsch, N. et al. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl Acad. Sci. USA111, 9762–9767 (2014). CASGoogle Scholar
- Brudno, Y. & Mooney, D. J. On-demand drug delivery from local depots. J. Control. Release219, 8–17 (2015). CASGoogle Scholar
- Lee, K. Y., Peters, M. C., Anderson, K. W. & Mooney, D. J. Controlled growth factor release from synthetic extracellular matrices. Nature408, 998–1000 (2000). CASGoogle Scholar
- Liu, T.-Y., Hu, S.-H., Liu, T.-Y., Liu, D.-M. & Chen, S.-Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir22, 5974–5978 (2006). CASGoogle Scholar
- Hu, S.-H., Liu, T.-Y., Liu, D.-M. & Chen, S.-Y. Nano-ferrosponges for controlled drug release. J. Control. Release121, 181–189 (2007). CASGoogle Scholar
- Zhao, X. et al. Active scaffolds for on-demand drug and cell delivery. Proc. Natl Acad. Sci. USA108, 67–72 (2011). CASGoogle Scholar
- Mitragotri, S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Dis.4, 255–260 (2005). CASGoogle Scholar
- Mitragotri, S., Blankschtein, D. & Langer, R. Ultrasound-mediated transdermal protein delivery. Science269, 850–853 (1995). CASGoogle Scholar
- Mann, B. K., Schmedlen, R. H. & West, J. L. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials22, 439–444 (2001). CASGoogle Scholar
- Kolate, A. et al. PEG — a versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release192, 67–81 (2014). CASGoogle Scholar
- Ehrbar, M. et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res.94, 1124–1132 (2004). CASGoogle Scholar
- Traub, S. et al. The promotion of endothelial cell attachment and spreading using FNIII10 fused to VEGF-A 165. Biomaterials34, 5958–5968 (2013). CASGoogle Scholar
- Van Hove, A. H., Beltejar, M.-J. G. & Benoit, D. S. Development and in vitro assessment of enzymatically-responsive poly(ethylene glycol) hydrogels for the delivery of therapeutic peptides. Biomaterials35, 9719–9730 (2014). CASGoogle Scholar
- Greenwald, R. B. et al. Controlled release of proteins from their poly(ethylene glycol) conjugates: drug delivery systems employing 1, 6-elimination. Bioconjugate Chem.14, 395–403 (2003). CASGoogle Scholar
- Schneider, E. L., Henise, J., Reid, R., Ashley, G. W. & Santi, D. V. Hydrogel drug delivery system using self-cleaving covalent linkers for once-a-week administration of exenatide. Bioconjugate Chem.27, 1210–1215 (2016). CASGoogle Scholar
- Shah, N. J. et al. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl Acad. Sci. USA111, 12847–12852 (2014). CASGoogle Scholar
- Macdonald, M. L. et al. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials32, 1446–1453 (2011). CASGoogle Scholar
- Silva, E. A. & Mooney, D. J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials31, 1235–1241 (2010). CASGoogle Scholar
- Kolambkar, Y. M. et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials32, 65–74 (2011). CASGoogle Scholar
- Martino, M. M. et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science343, 885–888 (2014). Growth factors were engineered to bind strongly to the extracellular matrix, which led to superior tissue repair and decreased side effects in the treatment of diabetic wounds, compared with the wild-type proteins, which have low affinity to the extracellular matrix.CASGoogle Scholar
- Pike, D. B. et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials27, 5242–5251 (2006). CASGoogle Scholar
- Freeman, I., Kedem, A. & Cohen, S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials29, 3260–3268 (2008). CASGoogle Scholar
- Freudenberg, U. et al. Heparin desulfation modulates VEGF release and angiogenesis in diabetic wounds. J. Control. Release220, 79–88 (2015). CASGoogle Scholar
- Thatiparti, T. R., Shoffstall, A. J. & von Recum, H. A. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials31, 2335–2347 (2010). CASGoogle Scholar
- Zhang, P., Cheetham, A. G., Lin, Y.-a. & Cui, H. Self-assembled Tat nanofibers as effective drug carrier and transporter. ACS Nano7, 5965–5977 (2013). CASGoogle Scholar
- Soukasene, S. et al. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano5, 9113–9121 (2011). CASGoogle Scholar
- Jensen, B. E., Dávila, I. & Zelikin, A. N. Poly(vinyl alcohol) physical hydrogels: matrix-mediated drug delivery using spontaneously eroding substrate. J. Phys. Chem. B120, 5916–5926 (2016). CASGoogle Scholar
- Mateen, R. & Hoare, T. Injectable, in situ gelling, cyclodextrin–dextran hydrogels for the partitioning-driven release of hydrophobic drugs. J. Mater. Chem. B2, 5157–5167 (2014). CASGoogle Scholar
- Kearney, C. J. & Mooney, D. J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater.12, 1004–1017 (2013). CASGoogle Scholar
- Alconcel, S. N., Baas, A. S. & Maynard, H. D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem.2, 1442–1448 (2011). CASGoogle Scholar
- Fishburn, C. S. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J. Pharm. Sci.97, 4167–4183 (2008). CASGoogle Scholar
- Lee, S., Greenwald, R. B., McGuire, J., Yang, K. & Shi, C. Drug delivery systems employing 1, 6-elimination: releasable poly(ethylene glycol) conjugates of proteins. Bioconjugate Chem.12, 163–169 (2001). CASGoogle Scholar
- Cheetham, A. G., Ou, Y.-C., Zhang, P. & Cui, H. Linker-determined drug release mechanism of free camptothecin from self-assembling drug amphiphiles. Chem. Commun.50, 6039–6042 (2014). CASGoogle Scholar
- Jo, Y. S., Gantz, J., Hubbell, J. A. & Lutolf, M. P. Tailoring hydrogel degradation and drug release via neighboring amino acid controlled ester hydrolysis. Soft Matter5, 440–446 (2009). CASGoogle Scholar
- Geng, H., Song, H., Qi, J. & Cui, D. Sustained release of VEGF from PLGA nanoparticles embedded thermo-sensitive hydrogel in full-thickness porcine bladder acellular matrix. Nanoscale Res. Lett.6, 1–8 (2011). Google Scholar
- Lee, J. & Lee, K. Y. Injectable microsphere/hydrogel combination systems for localized protein delivery. Macromol. Biosci.9, 671–676 (2009). CASGoogle Scholar
- Johnston, C. T., Premachandra, G. S., Szabo, T., Lok, J. & Schoonheydt, R. A. Interaction of biological molecules with clay minerals: a combined spectroscopic and sorption study of lysozyme on saponite. Langmuir28, 611–619 (2011). Google Scholar
- Dawson, J. I. & Oreffo, R. O. Clay: new opportunities for tissue regeneration and biomaterial design. Adv. Mater.25, 4069–4086 (2013). CASGoogle Scholar
- Takahashi, T., Yamada, Y., Kataoka, K. & Nagasaki, Y. Preparation of a novel PEG–clay hybrid as a DDS material: dispersion stability and sustained release profiles. J. Control. Release107, 408–416 (2005). CASGoogle Scholar
- Abdurrahmanoglu, S. & Okay, O. Rheological behavior of polymer-clay nanocomposite hydrogels: effect of nanoscale interactions. J. Appl. Polym. Sci.116, 2328–2335 (2010). CASGoogle Scholar
- Appel, E. A. et al. Exploiting electrostatic interactions in polymer–nanoparticle hydrogels. ACS Macro Lett.4, 848–852 (2015). CASGoogle Scholar
- Khaled, S. Z. et al. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials87, 57–68 (2016). CASGoogle Scholar
- Wichterle, O. & Lim, D. Hydrophilic gels for biological use. Nature185, 117–118 (1960). Google Scholar
- Ritger, P. L. & Peppas, N. A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release5, 23–36 (1987). CASGoogle Scholar
- Ritger, P. L. & Peppas, N. A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release5, 37–42 (1987). CASGoogle Scholar
- Schmidt, J. J., Rowley, J. & Kong, H. J. Hydrogels used for cell-based drug delivery. J. Biomed. Mater. Res. A87, 1113–1122 (2008). Google Scholar
- Fischbach, M. A., Bluestone, J. A. & Lim, W. A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl. Med.5, 179ps177 (2013). Google Scholar
- Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol.25, 1015–1024 (2007). CASGoogle Scholar
- Ballios, B. G. et al. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell Rep.4, 1031–1045 (2015). CASGoogle Scholar
- Robey, T. E., Saiget, M. K., Reinecke, H. & Murry, C. E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol.45, 567–581 (2008). CASGoogle Scholar
- Rustad, K. C. et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials33, 80–90 (2012). CASGoogle Scholar
- Lim, F. & Sun, A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science210, 908–910 (1980). CASGoogle Scholar
- Trivedi, N. et al. Islets in alginate macrobeads reverse diabetes despite minimal acute insulin secretory responses. Transplantation71, 203–211 (2001). CASGoogle Scholar
- Wang, N., Adams, G., Buttery, L., Falcone, F. H. & Stolnik, S. Alginate encapsulation technology supports embryonic stem cells differentiation into insulin-producing cells. J. Biotechnol.144, 304–312 (2009). CASGoogle Scholar
- Liras, A. Future research and therapeutic applications of human stem cells: general, regulatory, and bioethical aspects. J. Transl. Med.8, 131 (2010). Google Scholar
- Ma, M. et al. Core–shell hydrogel microcapsules for improved islets encapsulation. Adv. Healthc. Mater.2, 667–672 (2013). CASGoogle Scholar
- Parisi-Amon, A., Mulyasasmita, W., Chung, C. & Heilshorn, S. C. Protein-engineered injectable hydrogel to improve retention of transplanted adipose-derived stem cells. Adv. Healthc. Mater.2, 428–432 (2013). CASGoogle Scholar
- Roche, E. T. et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials35, 6850–6858 (2014). CASGoogle Scholar
- Levit, R. D. et al. Cellular encapsulation enhances cardiac repair. J. Am. Heart Assoc.2, e000367 (2013). Google Scholar
- Newland, B. et al. Tackling cell transplantation anoikis: an injectable, shape memory cryogel microcarrier platform material for stem cell and neuronal cell growth. Small11, 5047–5053 (2015). CASGoogle Scholar
- Alsberg, E., Anderson, K., Albeiruti, A., Franceschi, R. & Mooney, D. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dental Res.80, 2025–2029 (2001). CASGoogle Scholar
- Lin, C.-C., Raza, A. & Shih, H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials32, 9685–9695 (2011). CASGoogle Scholar
- Rowley, J. A. & Mooney, D. J. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res.60, 217–223 (2002). CASGoogle Scholar
- Bidarra, S. J. et al. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials32, 7897–7904 (2011). CASGoogle Scholar
- Burdick, J. A. & Anseth, K. S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials23, 4315–4323 (2002). CASGoogle Scholar
- Benoit, D. S., Schwartz, M. P., Durney, A. R. & Anseth, K. S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater.7, 816–823 (2008). CASGoogle Scholar
- Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell126, 677–689 (2006). CASGoogle Scholar
- Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater.15, 326–334 (2015). Google Scholar
- Shin, J.-W. & Mooney, D. J. Improving stem cell therapeutics with mechanobiology. Cell Stem Cell18, 16–19 (2016). CASGoogle Scholar
- Alsberg, E. et al. Regulating bone formation via controlled scaffold degradation. J. Dental Res.82, 903–908 (2003). CASGoogle Scholar
- Griffin, D. R., Weaver, W. M., Scumpia, P. O., Di Carlo, D. & Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater.14, 737–744 (2015). CASGoogle Scholar
- Stevens, K. R., Miller, J. S., Blakely, B. L., Chen, C. S. & Bhatia, S. N. Degradable hydrogels derived from PEG-diacrylamide for hepatic tissue engineering. J. Biomed. Mater. Res. A103, 3331–3338 (2015). CASGoogle Scholar
- Schlegel, P. N. & Group, H. S. Efficacy and safety of histrelin subdermal implant in patients with advanced prostate cancer. J. Urol.175, 1353–1358 (2006). CASGoogle Scholar
- Jaklenec, A., Stamp, A., Deweerd, E., Sherwin, A. & Langer, R. Progress in the tissue engineering and stem cell industry “are we there yet?”. Tissue Eng. Part B Rev.18, 155–166 (2012). Google Scholar
- Wurm, A., Nogler, M., Ammann, C. G. & Coraça-Huber, D. C. Effect of storage temperature and antibiotic impregnation on the quantity of bone morphogenetic protein seven in human bone grafts. Int. Orthop.38, 1513–1517 (2014). Google Scholar
- Spiller, K. L. & Vunjak-Novakovic, G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv. Transl. Res.5, 101–115 (2015). CASGoogle Scholar
- Hunziker, E. et al. Translation from research to applications. Tissue Eng.12, 3341–3364 (2006). CASGoogle Scholar
- Chen, R. R., Silva, E. A., Yuen, W. W. & Mooney, D. J. Spatio–temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res.24, 258–264 (2007). Google Scholar
- Kanczler, J. M. et al. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials31, 1242–1250 (2010). CASGoogle Scholar
- Basmanav, F. B., Kose, G. T. & Hasirci, V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials29, 4195–4204 (2008). Google Scholar
- Kearney, C. J. et al. Switchable release of entrapped nanoparticles from alginate hydrogels. Adv. Healthc. Mater.4, 1634–1639 (2015). CASGoogle Scholar
- Brudno, Y. et al. Refilling drug delivery depots through the blood. Proc. Natl Acad. Sci. USA111, 12722–12727 (2014). A new paradigm of refilling hydrogel drug depots that are already present in the body was presented, and the utility of highly specific drug–polymer interactions for this application was also demonstrated.CASGoogle Scholar
- Brudno, Y. et al. In vivo targeting through click chemistry. ChemMedChem.10, 617–620 (2015). CASGoogle Scholar
- Saltzman, W. M. & Radomsky, M. L. Drugs released from polymers: diffusion and elimination in brain tissue. Chem. Eng. Sci.46, 2429–2444 (1991). CASGoogle Scholar
- Weiser, J. R. & Saltzman, W. M. Controlled release for local delivery of drugs: barriers and models. J. Control. Release190, 664–673 (2014). This review provides a comprehensive overview of mathematical models for controlled drug release, highlighting the effect of tissue barriers on drug transport in the body.CASGoogle Scholar
- Santini, J. T., Cima, M. J. & Langer, R. A controlled-release microchip. Nature397, 335–338 (1999). CASGoogle Scholar
- Grayson, A. C. R. et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat. Mater.2, 767–772 (2003). CASGoogle Scholar
- Santini, J. T. Jr, Richards, A. C., Scheidt, R., Cima, M. J. & Langer, R. Microchips as controlled drug-delivery devices. Angew. Chem. Int. Ed.39, 2396–2407 (2000). CASGoogle Scholar
- Lin, S. et al. Stretchable hydrogel electronics and devices. Adv. Mater.28, 4497–4505 (2016). CASGoogle Scholar
- Flory, P. J. & Rehner, J. Statistical mechanics of cross-linked polymer networks II Swelling. J. Chem. Phys.11, 521–526 (1943). CASGoogle Scholar
- Kuijpers, A. et al. Characterization of the network structure of carbodiimide cross-linked gelatin gels. Macromolecules32, 3325–3333 (1999). CASGoogle Scholar
- Anseth, K. S., Bowman, C. N. & Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials17, 1647–1657 (1996). CASGoogle Scholar
- Koshy, S. T. et al. Click-crosslinked injectable gelatin hydrogels. Adv. Healthc. Mater.5, 541–547 (2016). CASGoogle Scholar
- Li, J. Y., Hu, Y. H., Vlassak, J. J. & Suo, Z. G. Experimental determination of equations of state for ideal elastomeric gels. Soft Matter8, 8121–8128 (2012). CASGoogle Scholar
- Hu, Y. H., Zhao, X. H., Vlassak, J. J. & Suo, Z. G. Using indentation to characterize the poroelasticity of gels. Appl. Phys. Lett.96, 121904 (2010). Google Scholar
- Drury, J. L., Dennis, R. G. & Mooney, D. J. The tensile properties of alginate hydrogels. Biomaterials25, 3187–3199 (2004). CASGoogle Scholar
- Adhikari, B. & Banerjee, A. Short peptide based hydrogels: incorporation of graphene into the hydrogel. Soft Matter7, 9259–9266 (2011). CASGoogle Scholar
- Waters, D. J. et al. Morphology of photopolymerized end-linked poly(ethylene glycol) hydrogels by small-angle X-ray scattering. Macromolecules43, 6861–6870 (2010). CASGoogle Scholar
- Krogstad, D. V. et al. Small angle neutron scattering study of complex coacervate micelles and hydrogels formed from ionic diblock and triblock copolymers. J. Phys. Chem. B118, 13011–13018 (2014). CASGoogle Scholar
- Zhang, X., Hansing, J., Netz, R. R. & DeRouchey, J. E. Particle transport through hydrogels is charge asymmetric. Biophys. J.108, 530–539 (2015). CASGoogle Scholar
- Fatin-Rouge, N., Starchev, K. & Buffle, J. Size effects on diffusion processes within agarose gels. Biophys. J.86, 2710–2719 (2004). CASGoogle Scholar
Acknowledgements
This work was supported by the government under R01DE0130333 awarded by the US National Institute of Dental & Craniofacial Research of the National Institutes of Health, and award A21448 from Novartis Pharmaceuticals Corporation. The authors thank L. Gu and A. Göpferich for discussions.









